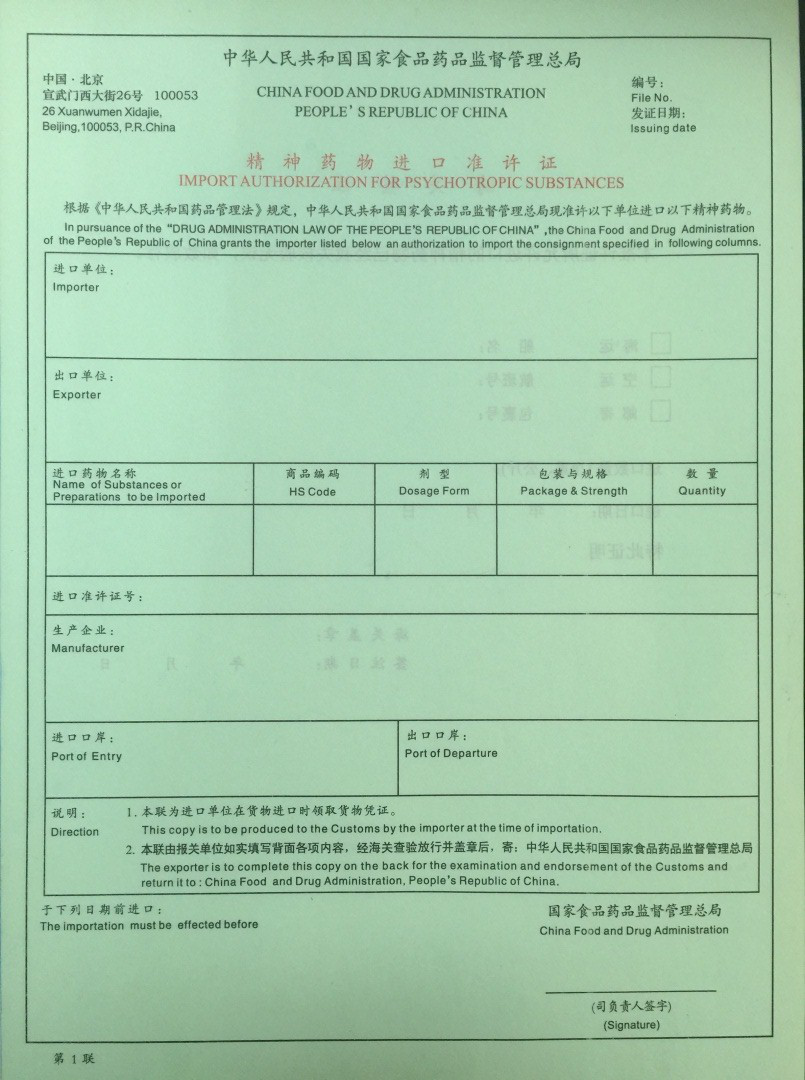
Import and Export of Narcotic Drugs and Psychotropic Substances License Approval and Approval Service Guide
I, The Scope of Application
This guideline applies to the application and handling of the issuance of import and export permits for narcotic drugs and psychotropic substances.
II, The Project Information
(1) Project Name: Approval of the Import and Export Permit for Narcotic Drugs and Psychotropic Drugs
(2) Type of matter review: post-trial approval
(3) Project code: 30015
III, The Basis for Handling
Article 45 of the Drug Administration Law: Import and export of narcotic drugs and psychotropic drugs within the scope of state regulations must be accompanied by the Import Permit and the Export Permit issued by the drug regulatory authority under the State Council.
IV, The Receiving Agency
Food and Drugs Inspection and Inspection Center of the State Drug Administration.
V, Decision Body
State Drug Administration.
VI, The Number of Approvals
No limit.
VII, Application Conditions
Applicants’ conditions can be found in the application materials for the qualification requirements of import and export units.
VIII, Application Materials
(1) Export approval for narcotic drugs and psychotropic substances
1. Application materials catalog
(1) Application form for special drug exports;
(2) Import permit (original) provided by the competent authority of the anesthetic (mental) drug in the importing country or region;
(3) A copy of the purchase contract or order;
(4) A copy of the export contract or order;
(5) If the exported medicine is a variety that has been approved for production by a domestic pharmaceutical production enterprise, a copy of the Pharmaceutical Production License, the Business License of the Enterprise Legal Person and the approval certificate of the pharmaceutical shall be provided;
If the export drug is a domestic enterprise that accepts the products commissioned by the overseas enterprise, it shall provide a copy of the certification documents approved by the State Drug Administration.
(6) A copy of the “Business License” and “Registration Form for Foreign Trade Operators” of the export enterprise.
2. General requirements for filing materials
(1) The application items and contents are accurate, the application materials are complete and clear, and the A4 specification paper is printed or copied on one side.
(2) Fill in the specifications and prove that the documents are valid.
(3) The electronic version of the application form should be sent to slzx@cfda.gov.cn when mailing or submitting application materials.
(4) The above-mentioned various types of copies shall be stamped with the official seal of the exporting unit.
3. Specific requirements for the application materials:
(1) “Special Drug Export Application Form”
1) The application form must be filled in accurately and in a standardized manner, and meet the requirements of the instructions for filling out the form.
2) The name and address of the importing unit in the application form must be the same as the name of the unit and the registered address in the submitted qualification certificate.
3) The application form must indicate the specific import and export port, no more than two.
(2) Import permit (original) provided by the competent authority of the anesthetic (mental) drug in the importing country or region
Individual countries may not require import permits for the import of individual varieties, which is subject to adjustments provided by the United Nations Board. Exports of individual varieties are also required to provide a letter of guarantee from the importer. If the import permit is non-English, it must be translated in Chinese or English and notarized.
(3) Copy of purchase contract or order
The contract or order must be signed and stamped by the responsible person of the buyer and the seller.
(4) Copy of export contract or order
The contract or order shall be signed by the representatives of the buyer and the seller and shall indicate the name and position of the signatory.
(2) Approval for import of clinical use of narcotic drugs and psychotropic drugs
1. Application materials catalog
(1) Application form for special drug import;
(2) A copy of the purchase contract or order;
(3) A copy of the “Imported Drug Registration Certificate” or “Pharmaceutical Product Registration Certificate” (original or copy) (instructions for teaching, scientific research, and special import of clinical products may not be provided);
(4) A copy of the “Business License” and “Registration Form for Foreign Trade Operators” of the importing unit; the raw materials and preparation intermediates (including domestic packaging preparations) required for the pharmaceutical manufacturer to import the enterprise shall be submitted for business. A copy of the License and the Pharmaceutical Production License;
(5) If the exporting unit is the sales agent of the drug, it must also provide the documentary evidence of the legal qualification of the exporting unit, the notarized document and its Chinese translation.
Narcotic drugs and psychotropic drugs imported for medical use for the first time must be subject to technical review before they can apply for the “Anesthesia (spiritual) drug import permit”.
2. General requirements for filing materials:
(1) The application items and contents are accurate, the application materials are complete and clear, and the A4 specification paper is printed or copied on one side.
(2) Fill in the specifications and prove that the documents are valid.
(3) The electronic version of the application form should be sent to slzx@cfda.gov.cn when mailing or submitting application materials.
(4) The above-mentioned various types of copies shall be stamped with the official seal of the importing unit.
3. Specific requirements for the application materials:
(1) “Special Drug Import Application Form”
1) The application form must be filled in accurately and in a standardized manner, and meet the requirements of the instructions for filling out the form.
2) The name and address of the importing unit to be filled in the application form must be the same as the name of the unit and the registered address in the submitted qualification certificate.
3) The application form must indicate the specific import and export port, no more than two.
(2) Copy of purchase contract or order
The contract or order shall be signed by the responsible person of the buyer and the seller and affixed with the official seal, and the name and position of the signatory shall be indicated.
(3) Approval of import of narcotic drugs and psychotropic drugs for teaching and research
1. Application materials catalog
(1) Application form for special drug import;
(2) A copy of the purchase contract or order;
(3) Proof of documents, notarized documents and Chinese translations (standards and reference imports may be provided) issued by the drug regulatory agency of the country or region where the drug is allowed to go on sale;
(4) If the exporting unit is the sales agent of the drug, it must also provide the supporting documents, notarized documents and Chinese translations of the legal qualifications of the exporting unit;
(5) The documentary evidence of the legal qualifications of the domestic use unit, the basis for the measurement of the amount of the drug used, and the letter of guarantee for the legal use and management of the drug issued by the user;
(6) The approval documents of the corresponding scientific research projects or the approval documents of the corresponding competent authorities;
(7) If entrusting other importing units to act as agents, it is also required to entrust a copy of the agency agreement and a copy of the “Business License” and “Registration Form for Foreign Trade Operators” of the importing unit.
2. General requirements for filing materials:
(1) The application items and contents are accurate, the application materials are complete and clear, and the A4 specification paper is printed or copied on one side.
(2) Fill in the specifications and prove that the documents are valid.
(3) The electronic version of the application form should be sent to slzx@cfda.gov.cn when mailing or submitting application materials.
(4) The above-mentioned various types of copies shall be stamped with the official seal of the importing unit.
3. Specific requirements for the application materials:
(1) “Special Drug Import Application Form”
1) The application form must be filled in accurately and in a standardized manner, and meet the requirements of the instructions for filling out the form. The official seal of the unit must be affixed.
2) The name and address of the importing unit to be filled out in the application form must be the same as the name of the unit and the registered address in the submitted qualification certificate.
3) The application form must indicate the specific import and export port, no more than two.
(2) Copy of purchase contract or order
The contract or order shall be signed by the responsible person of the buyer and the seller and affixed with the official seal, and the name and position of the signatory shall be indicated.
(4) Submission of application materials
Applicants can submit materials through window submission, mailing, etc.
The attachment is the Import of Narcotic Drugs and Psychotropic Substances License